
So the molecular formula of cyclopropane is C 3H 6.Īuthor: Fred Senese Chemistry Online! How can I find the molecular formula of a gas from experimental data?Ĭomments & questions to Revised 02/23/18. In this case, the molecular formula weight divided by the empirical formula weight is 42.0 20768/14.026 94 = 3. Is repeated to make the molecular formula. This number tells you how many times the empirical formula Divide the molecular weight by the formula weight.Find the empirical formula weight by adding up the weights of the atoms in the empirical formula.įor CH 2, the formula weight is 12.011 + 2 × 1.00797 = 14.026 94 g/mol.The molecular weight of the gas is grams of gas (1.56 g) divided by moles of gas:ġ.56 g ÷ 0.0371 245 mol = 42.0 20768 g/mol.N = (0.984 atm)×(1 L)/(0.08206 L atm mol -1 K -1 × 323 K) Empirical Formula By making use of the molar mass from the periodic table, change the mass of every element to moles.

That means the percents can be expresse as mass: Na. Calculate the number of moles of gas from the given pressure, volume, and temperature. Calculate empirical formula when given percent composition data 1) Assume 100 g of the compound is present. Like molecular formulas, empirical formulas are not unique and can describe a number of different chemical structures or isomers.To find the molecular formula, you need to find the molecular weight of the compound. 1.98 83223 rounds to 2, so the empirical formula These are the subscripts in the empirical formula. Round the mole ratios to the nearest whole number (or simple fraction).In this case, C is present in the smallar molariĪmount so the ratios are 1 for carbon and 14.1 8693 ÷ 7.13 51261 = 1.98 83223 for hydrogen.

These are intermediate results, so don't round them.ĭivide moles of each element by the smallest molar amount.
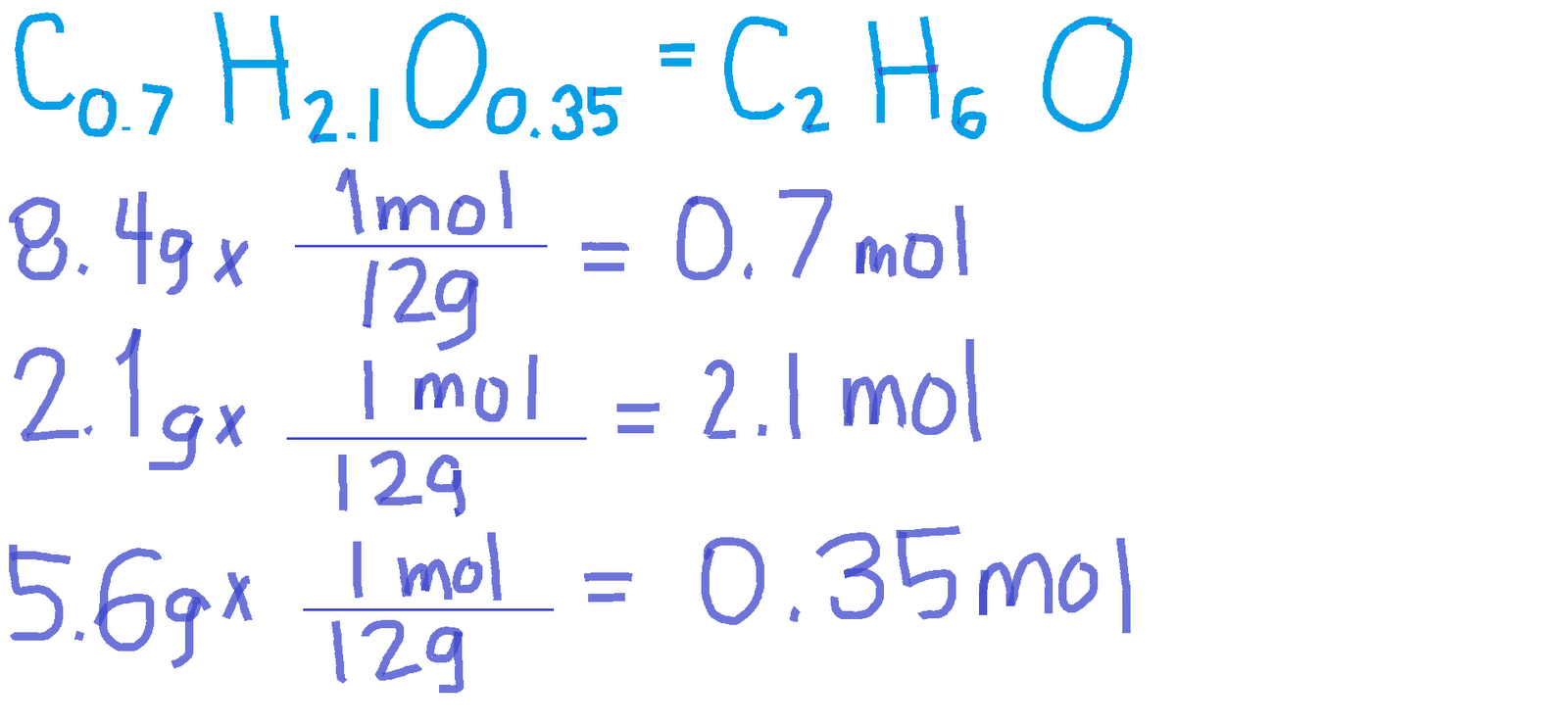
Notice that I haven't rounded off the mole amounts to the correct number of significant digits (the nonsignificant figures are written as subscripts). So you have 85.7 g C and 14.3 g H in a hundred-gram sample of cyclopropane. Write the mass percent as a mass Mass percent is just mass per 100 g sample.What is the empirical and molecular formula of the cyclopropane?įirst, use the element mass percents to obtain the empirical formula of the cyclopropane. Formula Used: (i) atomic-ratio (compound - percentage) / (atomic mass) (from periodic table) Where, atomic ratio - atoms of one kind to another kind. At 50.0 C and 0.984 atm pressure 1.56g cyclopropane has a volume of 1 L. Cyclopropane contains 85.7% C, and 14.3% H by mass. How can I find the molecular formula of a gas from experimental data?Ī mixture of cyclopropane gas and oxygen is used in an anaesthetic.


 0 kommentar(er)
0 kommentar(er)
